Risk reduction and early intervention in lymphoedema

Helen Boursinos, Gillian Buckley, Lizzie Eastwood, Lisa Parry and Dr Teresa Lee present five discussion points about the strategies physiotherapists can use to prevent and manage lymphoedema and to reduce its impact on patients.
1. Identifying risk factors reduces the likelihood of developing lymphoedema
A number of risk factors increase a patient’s chance of developing lymphoedema.
The identification of individual risk factors and education on risk-reducing strategies are important in the overall management of patients at risk of lymphoedema (Woods 2019).
For patients at risk of cancer-related lymphoedema, increased body mass index is a well-established risk factor (Ridner 2011).
Other risk factors include advanced cancers, extensive cancer surgery, a high number of lymph nodes removed and surgical complications such as wound infection and seromas (Toyserkani 2017).
Cancer treatment, specifically radiotherapy to nodal basins and taxane-based chemotherapy, can result in damage to the lymphatic vessels, which also increases the lifelong risk of developing lymphoedema (Koelmeyer 2022).
In the non-cancer population, any chronic oedema that stems from venous insufficiency, trauma, inflammatory conditions or cellulitis can increase the risk of lymphoedema (Woods 2019).

Other oedemas related to cardiac or renal dysfunction can also overload the lymphatic system, leading to increased lymphoedema risk (Woods 2019).
Hormone-mediated tissue inflammation associated with obesity has been shown to cause lymphatic vessel damage (Bertsch 2018) and obesity-related lymphoedema is becoming a more common presentation.
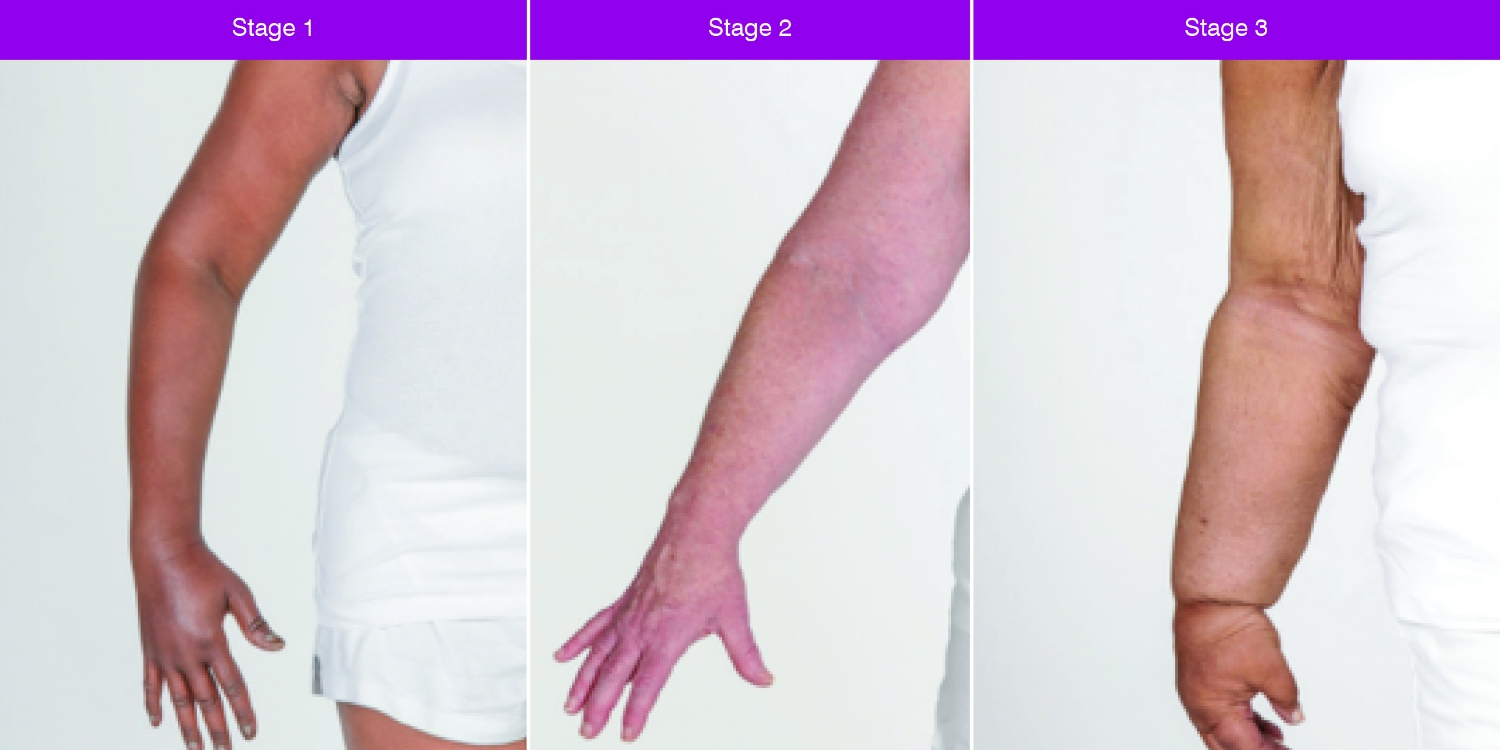
2. Early intervention can prevent progression to chronic lymphoedema
For more than a decade, researchers and clinicians have been recommending a prospective surveillance model for the detection of breast cancer-related lymphoedema (Stout 2012).
A growing body of evidence now supports prospective screening to allow for early diagnosis and intervention (McLaughlin 2020).
This early intervention, ideally at a subclinical stage, can prevent progression to a more chronic form of lymphoedema.
Baseline pre-treatment assessment and regular screening by a lymphoedema therapist are considered the best model of care for those at risk of cancer-related lymphoedema.
In a large, multicentre randomised controlled trial published in 2021 (Ridner 2021), including Australian patients, researchers used bioimpedance spectroscopy to identify subclinical extracellular fluid change in patients being treated for breast cancer.
They found that coupled with early intervention, including the prescription of a well-fitting compression garment, this reduced progression to breast cancer-related lymphoedema in 95 per cent of cases.
The Australasian Lymphology Association (Dylke 2019) recommends that all breast cancer patients, particularly those at higher risk, have access to education and a prospective monitoring program to measure changes indicative of swelling, including preoperative measurements.
Monitoring should continue postoperatively and at regular intervals for at least two years.
3. Skin health is integral to lymphoedema management
The skin is the body’s first line of defence against external pathogens and any opening or break in the skin provides a portal of entry for microorganisms.
This is an important consideration for patients with lymphoedema.
To promote skin integrity and minimise the risk of infection, skin should be cleaned, carefully dried and moisturised with an emollient every day (Nowicki 2013).
Regular inspection of the skin for dryness, abrasions, cuts, excoriation or signs of bacterial or fungal infection is also recommended (Linnitt 2012) and any trauma to the skin should be treated antiseptically.
Strategies to protect the skin from injury can also prevent a portal of entry for infection (Ridner 2011).

This may include the use of sunscreen, insect repellent and protective clothing when performing activities that commonly cause skin injury.
Approximately one-third of people with lymphoedema experience cellulitis (Todd 2013).
The symptoms of cellulitis are fever, skin rubor with ill-defined margins and tenderness.
Episodes generally require a course of antibiotics and if infections are recurrent, the patient may require long- term prophylactic antibiotics (Australasian Lymphology Association 2015).
4. Patients with or at risk of lymphoedema should be encouraged to exercise
Exercise is recommended for patients who have lymphoedema or are at risk of developing it.
Both aerobic exercise and resistance training are safe and beneficial, while flexibility exercises help to minimise the skin scarring and joint contracture that may reduce lymph flow.
Patients who have had breast cancer surgery and lymph node removal are encouraged to exercise their affected limb (Hayes 2022, Sayegh 2017, Wu 2021, Schmitz 2010, National Lymphedema Network Medical Advisory Committee 2012).
Physiotherapists should consider a patient’s medical history before the patient begins an exercise program.
Exercise should be increased slowly, with sufficient rest intervals between sets, avoiding weights that wrap tightly around a limb and clothing that causes constriction.
It is important for patients to maintain hydration and avoid extreme heat (National Lymphedema Network Medical Advisory Committee 2011).
A common question asked by patients is whether they need to wear compression garments while performing exercise.
This is something that warrants discussion between the physiotherapist and patient with respect to the patient’s lymphoedema risk profile, the chosen exercise and the patient’s ability to exercise with compression in place (National Lymphedema Network Medical Advisory Committee 2012).
All patients need to be encouraged and supported to report any adverse effects when exercising so that their exercise program can be adapted accordingly.
Exercising and maintaining a healthy body weight are important for reducing the risk of lymphoedema and managing the condition (National Lymphedema Network Medical Advisory Committee 2012, Ridner 2011).
5. Compression can reduce and help prevent lymphoedema
Compression is an effective way of addressing lymphoedema and reducing its progression.
It facilitates an increase in tissue pressure that assists the movement of fluid into the collecting lymphatics and reduces excess filtration out of the blood vessels.
Patients with mild lymphoedema may be fitted into a compression garment at diagnosis, while those with moderate to severe lymphoedema may require a course of multilayer bandaging for optimal lymphoedema reduction prior to being fitted with a garment.
Velcro wraps and sequential intermittent compression pumps can also lead to significant lymphoedema reduction.

Compression garments can be used prophylactically by high-risk patients after lymph node removal to reduce their likelihood of developing lymphoedema (Paramanandam 2022).
Careful consideration of garment choice has an impact on patients’ comfort and adherence.
Lifelong use of compression garments may be required for lymphoedema management.
However, some patients with early lymphoedema can progressively wean off compression garments if their bioimpedance readings fall back within the normal range over time (Stout Gergich 2008, Ridner 2019).
The aim of physiotherapy management is to work out the minimum amount of compression required to manage each patient’s lymphoedema.
Other therapies such as manual lymphatic drainage and low-level laser may also be helpful when used in combination with compression.
Click here for an infographic poster version of this article.
>> Helen Boursinos APAM is a lymphoedema therapist at Monash Health and director of Life Flow Physiotherapy in Melbourne. Helen is a member of the Australasian Lymphology Association and a committee member of the Victorian branch of the APA Cancer, Palliative Care and Lymphoedema group.
>> Gillian Buckley APAM MACP is an APA Lymphoedema Physiotherapist who has worked in lymphoedema management and post-cancer physiotherapy care for more than 20 years. Gillian works in private practice in Melbourne.
>> Lizzie Eastwood APAM is the clinical lead oncology physiotherapist at Hollywood Private Hospital in Perth. Lizzie has completed advanced training in lymphoedema, Pinc and Steel cancer rehabilitation and oncology scar therapy. She is a member of the Australasian Lymphology Association and the Western Australian branch of the APA Cancer, Palliative Care and Lymphoedema group.
>> Lisa Parry APAM is a certified lymphoedema and Pinc cancer rehabilitation physiotherapist working at the Mater Hospital Brisbane. Lisa has been a committee member of the Queensland branch of the APA Cancer, Palliative Care and Lymphoedema group since 2016 and is a member of Health Translation Queensland.
>> Dr Teresa Lee APAM MACP is an APA Cancer Physiotherapist and an APA Lymphoedema Physiotherapist. Teresa attained a PhD in physiotherapy rehabilitation of breast cancer patients in 2008. She works at Royal North Shore Hospital in Sydney and was the chair of the New South Wales branch of the APA Cancer, Palliative Care and Lymphoedema group from 2012 to 2019.
- References
-
Australasian Lymphology Association (ALA) Consensus Guideline: Management of Cellulitis in Lymphoedema, March 2015
Ammitzbøll, G., Kjær, T.K., Johansen, C., et al. 2019. Effect of progressive resistance training on health-related quality of life in the first year after breast cancer surgery – results from a randomized controlled trial, Acta Oncologica, 58:5, 665-672.
Ammitzbøll, G., Lanng, C., Kroman, N., Zerahn, B., Hyldegaard, O., et al. 2017. Progressive strength training to prevent Lymphoedema in the first year after breast Cancer - the LYCA feasibility study. Acta Oncol. Feb;56(2):360-366.
Bertsch T, Obesity-related lymphoedema- underestimated and undertreated, Phlebologie 2/2018.
Bloomquist, K., Karlsmark, T., Christensen, K.B., et al. 2014. Heavy resistance training and lymphedema: prevalence of breast cancer-related lymphedema in participants of an exercise intervention utilizing heavy load resistance training. Acta Oncol. (53):216–225.
Campbell, K. L., Winters-Stone, K.M., Wiskemann, J., May, A. M., et al. 2019. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Medicine and Science in Sports & Exercise., Vol. 51 (11): 2375–2390.
Cormier, J.N., Askew R.L., Mungovan, K.S., et al. 2010. Lymphoedema Beyond Breast Cancer: A Systematic Review and Meta-Analysis of Cancer-Related Secondary Lymphoedema. Cancer Month 00:1-12.
Dylke E 2019 ALA Position Statement Early detection of breast cancer-related lymphoedema. Australian LymphologyAssociationhttps://www.lymphoedema.org.au/public/7/files/Position%20Statements/ALA%20Position%20Statement_Early%20Detection%20of%20BCRL.pdf
Exercise. Position Statement of the National Lymphatic Network. 2013.
Fu MR, Ridner SH, Armer J. Post-breast cancer. Lymphedema: part 2. Am J Nurs 2009;109(8):34–41.
Gergich, N. L., L. A. Pfalzer, C. McGarvey, B. Springer, L. H. Gerber and P. Soballe (2008). "Preoperative assessment enables the early diagnosis and successful treatment of lymphedema." Cancer: Interdisciplinary International Journal of the American Cancer Society 112(12): 2809-2819.
Hayes SC, Singh B, Reul-Hirche H, Bloomquist K, Johansson K, Jönsson C, Plinsinga ML. The Effect of Exercise for the Prevention and Treatment of Cancer-Related Lymphedema: A Systematic Review with Meta-analysis. Med Sci Sports Exerc. 2022 Aug 1;54(8):1389-1399. doi: 10.1249/MSS.0000000000002918. Epub 2022 Mar 22. PMID: 35320145.
International Consensus, Best Practice for Management of Lymphoedema, London 2006 Australasian Lymphology Association: What is Lymphoedema
Kim M., Shin, K.H., Jung, S.Y., et al. 2016. Identification of prognostic risk factors for transient and persistent lymphedema after multimodal treatment for breast cancer. Cancer Res Treat. 48:1330–1337.
Koelmeyer L, Gaitatzis K, Dietrich M et al, Risk factors for breast cancer-related lymphoedema in patients undergoing 3 years of prospective surveillance with intervention, CancerSeptember 15, 2022
Kwan, M.L., Darbinian, J., Schmitz, K.H., et al. 2010. Risk Factors for Lymphoedema in a Prospective Breast Cancer Survivorship Study, the Pathways Study. Arch Surg 145 (11): 1055-1063.
Linnitt N, Mortimer PS, Hardy D. Skin care for people with lymphoedema fact sheet. London: Lymphoedema Support Network 2012
McLaughlin SA, Stout NL, Schaverien MV Avoid the swell: Advances in Lymphedema Prevention, Detection and Management. American Society of Clinical Oncology 2020 https://doi.org/10.1200/EDBK_280471
NLN Medical Advisory Committee. (2011, Dec). Position papers. National Lymphedema Network. Retrieved December 11, 2022, from https://lymphnet.org/position-papers
NLN Medical Advisory Committee. (2012, May). Position papers. National Lymphedema Network. Retrieved December 11, 2022, from https://lymphnet.org/position-papers
Nowicki J, Siviour A. Best practice skin care management in lymphoedema. Wound Practice and Research 2013;21(2):61–5.
Paramanandam, V. S., E. Dylke, G. M. Clark, A. A. Daptardar, A. M. Kulkarni, N. S. Nair, R. A. Badwe and S. L. Kilbreath (2022). "Prophylactic Use of Compression Sleeves Reduces the Incidence of Arm Swelling in Women at High Risk of Breast Cancer–Related Lymphedema: A Randomized Controlled Trial." Journal of Clinical Oncology 40(18): 2004-2012.
Ridner SH, Dietrich MS, Boyages J, Koelmeyer L, Elder E, Hughes TM, French J, Ngui N, Hsu J, Abramson VG, MooreA and Shah C A. 2021. Comparison of Bioimpedance Spectroscopy or Tape Measure Triggered Compression Intervention in Chronic Breast Cancer Lymphedema Prevention in Lymphatic Research and Biology https://doi.org/10.1089/lrb.2021.0084
Ridner SH, Dietrich MS, Stewart BR, Armer JM. Body mass index and breast cancer treatment-related lymphedema. Support Care Cancer. 2011 Jun;19(6):853-7. doi: 10.1007/s00520-011-1089-9. Epub 2011 Jan 16. PMID: 21240649; PMCID: PMC4480912.
Ridner, S. H., M. S. Dietrich, M. S. Cowher, B. Taback, S. McLaughlin, N. Ajkay, J. Boyages, L. Koelmeyer, S. M. DeSnyder and J. Wagner (2019). "A randomized trial evaluating bioimpedance spectroscopy versus tape measurement for the prevention of lymphedema following treatment for breast cancer: interim analysis." Annals of surgical oncology 26(10): 3250-3259.
Sayegh, H.E., Asdourian, M.S., Swaroop, M.N., et al. 2017. Diagnostic methods, risk factors, prevention, and management of breast cancer-related lymphedema: past, present, and future directions. Curr. Breast Canc. Rep., 9 (Suppl):1-11.
Schmitz KH, Ahmed RL, Troxel AB, Cheville A, Lewis-Grant L, Smith R, Bryan CJ, Williams-Smith CT, Chittams J. Weight lifting for women at risk for breast cancer-related lymphedema: a randomized trial. JAMA. 2010 Dec 22;304(24):2699-705. doi: 10.1001/jama.2010.1837. Epub 2010 Dec 8. PMID: 21148134.
Stout NL, Binkley JM, Schmitz KH, et al. A prospective surveillance model for rehabilitation for women with breast cancer. Cancer. 2012;118(Suppl):2191-2200Stout Gergich NL, Pfalzer LA, McGarvey C, Springer B, Gerber LH, Soballe P. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer. 2008 Jun 15;112(12):2809-19. doi: 10.1002/cncr.23494. PMID: 18428212.
Todd M. Chronic oedema: impact and management. Br J Nurs 2013;22(11):623–27.
Toyserkani N, Jørgensen M, Haugaard K, Sørensen J. Seroma indicates increased risk of lymphedema following breast cancer treatment: a retrospective cohort study. Breast. 2017; 32: 102-104. https://doi.org/ 0.1016/j.breast.2017.01.009
Woods M, Risk factors for the development of lymphoedema, British Journal of Nursing 2019, Vol 28, No.4: 219-222.
Wu X, Liu Y, Zhu D, Wang F, Ji J, Yan H. Early prevention of complex decongestive therapy and rehabilitation exercise for prevention of lower extremity lymphedema after operation of gynecologic cancer. Asian J Surg. 2021 Jan;44(1):111-115. doi: 10.1016/j.asjsur.2020.03.022. Epub 2020 May 10. PMID: 32402630.
© Copyright 2024 by Australian Physiotherapy Association. All rights reserved.