Not a cervicogenic headache?

If you treat people with headaches or those with hypermobility disorders, chances are you could come across a client with intracranial hypotension (IH) due to a spinal leak of cerebrospinal fluid (CSF) during your career.
Despite the significant knowledge you hold that could assist this client’s recovery, you can certainly be forgiven for not recognising that they had IH or even knowing anything about the condition.
Conversely, your alertness to the possibility of this diagnosis could significantly improve the management and outcomes for these clients and prevent them from facing prolonged, chronic, unnecessary disability.
Background
A condition involving headaches due to a leak of CSF after a lumbar puncture has been recognised since shortly after the lumbar puncture procedure was introduced in the late 19th century.
A similar presentation without a history of lumbar puncture was described as early as 1940, but the diagnosis of spontaneous spinal CSF leaks leading to IH only became common in the last few decades as improvements in imaging technology have assisted the recognition and diagnosis of the condition (Mokri 2001).
Despite this, diagnosis remains challenging, with a lack of awareness among most health professionals and low sensitivity of diagnostic procedures (including MRI, CT, direct measurement of CSF pressure during lumbar puncture and myelography all having significant rates of false negatives (Mokri 1999)).
Consequently, diagnosis is often significantly delayed. International studies from centres with a clinical focus on this disorder have reported an average of 13 months between the onset of symptoms and diagnosis for patients with this disorder (Schievink 2003).
A recent small Australian study suggested that patients will suffer symptoms of this disorder for an average of 2.7 years before receiving a diagnosis (McQueen et al 2019).
A growing number of neurologists, neurosurgeons, neuroradiologists and pain management specialists in Australia are beginning to recognise and treat the condition.
While effective treatment for the disorder is available, failure to address the condition can have serious consequences including subdural haemorrhages and an array of other neurological complications (Beck et al 1998, Pakiam et al 1999, Pleasure et al 1998, Schievink 2006).
IH that occurs spontaneously or as a result of trauma (as distinct from iatrogenically) from a leak of CSF in the spine is thought to affect approximately five in 100,000 people per year (approximately half the rate of spontaneous subarachnoid haemorrhages) (Schievink et al 2007).
A significant proportion of these patients have an underlying heritable disorder of connective tissue (HDCT) or hypermobility syndrome such as Ehlers Danlos or Marfan syndrome (Schievink et al 2004). The onset of symptoms may be associated with trauma of varying degrees such as a whiplash or fall, or there may be no identifiable precipitating event.
The cardinal symptom of IH is a headache that is typically described as worse when upright and improving on lying down. Significant variability in this pattern has, however, been described, particularly when the disorder is ongoing for some time. Patients may instead describe a headache that is worse during the latter part of the day and better on rising in the morning, or may fail to notice an orthostatic pattern at all, particularly if the issue is more chronic (Schievink et al 1996).
The headache is generally bilateral and may be diffuse or localised—most commonly to the occipital region but possibly frontal or temporal. It is often described as gradually increasing in intensity over minutes to hours of being upright, but can be instantaneous (Schievink 2006).
Patients typically also report significant cervical and thoracic pain and so may present to physiotherapists with a presentation in some respects overlapping with cervicogenic headache. Furthermore, at least one study has identified the presence of spinal CSF leaks in 66 per cent of a group of patients diagnosed as having chronic whiplash associated disorder (Ishikawa et al 2007).
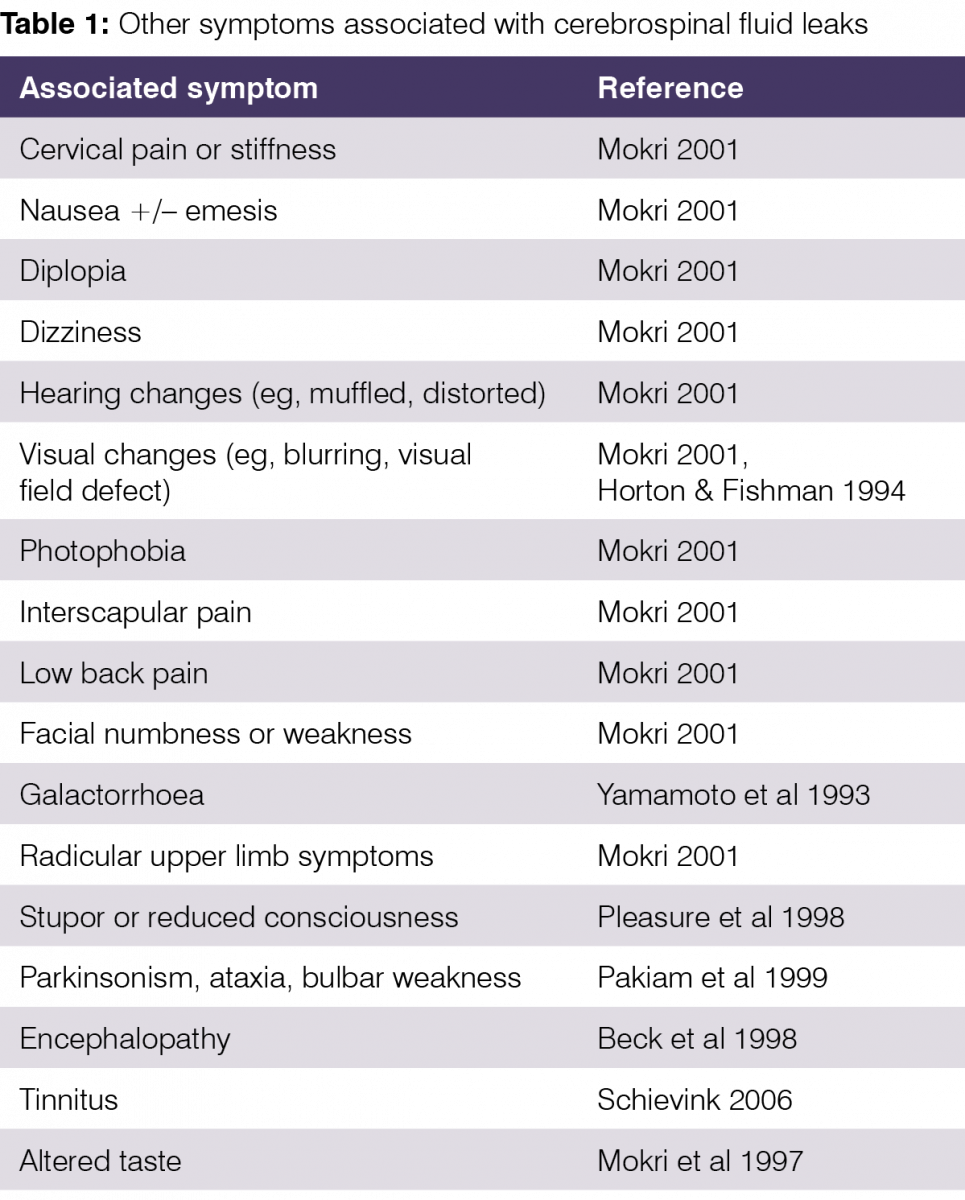
A vast range of other symptoms variably accompany the headache and spinal pain, including those in Table 1.
Patients with spinal CSF leaks leading to IH have been reported to have a range of different dural defects allowing the CSF to leak. These include absent or damaged nerve root sleeves, discrete tears of the dura, broadly attenuated areas of dura that weep CSF, and venous fistulas draining CSF directly into the venous system (Schievink 2006).
As a result of the increased losses of CSF over and above normal reabsorption, the patient’s body may be unable to maintain the usual volume and pressure of CSF. Therefore, the buoyancy which normally reduces the brain’s apparent weight from around 1500g to around 50g (Mokri 1999) is lost, and the brain can descend when the patient is in an upright position, sometimes giving the appearance of a Chiari malformation (Schievink 2006).
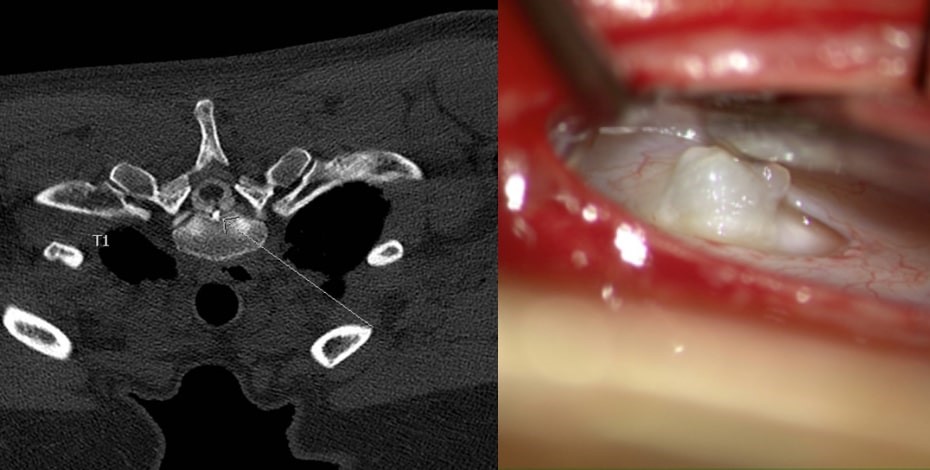
MRI and intraoperative image of the same patient showing a large bone spur and the defect where it perforated the dura. Images courtesy of Dr Scott Davies
The traction on innervated intracranial structures, and the distension of the cranial venous system due to increased blood volume compensating for the loss of CSF volume, are both thought to contribute to the symptoms of IH.
Other symptoms can arise from mechanical, vascular or other effects on brain structures such as the cranial nerves, diencephalon, and pituitary gland, or on inner ears, nerve roots or the meninges (Mokri 2001, Schievink 2006, Portier et al 2002) as the buoyancy, shock absorption, waste clearance and homeostatic functions of the CSF are potentially impacted by the loss of volume and pressure. The mechanisms at play are yet to be fully understood.
Management
Common treatment procedures for spinal CSF leaks that do not spontaneously heal with conservative measures (eg, bedrest) include epidural blood patching (EBP), fibrin glue patching, and surgical repairs of the dura. EBP, which is commonly trialled first, involves injection of autologous blood into the epidural space. In spontaneous leaks, larger volumes are often required than with iatrogenic leaks, commonly in the order of 20 to 30 millilitres.
Spontaneous leaks may require repeated patching before a sustained seal is achieved and if this is unsuccessful, fibrin glue or surgical repairs may be attempted where leak sites can be identified (Schievink 2006).
Given the underlying pathologies involved in spinal CSF leaks, there would seem to be much that physiotherapists could offer to assist in recovery, and yet there is little in the literature at this time to guide safe and effective physiotherapy management.
Until this situation changes, clinicians must rely on their knowledge of anatomy, physiology and pathology of related disorders and strong clinical reasoning.
Underlying principles to guide clinical intervention include protecting the healing tissue at the leak site, optimising tissue repair and mechanics of the spine, dura, and entire neural system, providing dethreatening information and addressing the likely deconditioning and comorbidities of the patient.
There are a wide range of factors that may be relevant to the recovery of any given client, other than the leak repair procedures undertaken.
Firstly, there is high likelihood of the presence of HDCT. This adds the possibility of impaired tissue healing, impaired proprioception, the presence of autonomic dysfunctions and a range of other disorders that present more commonly in the hypermobile population, along with the increased ranges of motion that the patient’s neural system may need to adapt to compared to a client of average mobility (Keer & Grahame 2003).
Rebound IH can occur for a period after successful sealing of a CSF leak (Schievink et al 1996). This reversal of intracranial pressure to a higher than normal level after sealing of a CSF leak can cause significant symptoms and disability for the client with a potential reversal of their orthostatic pattern to intolerance of lying down.
The raised CSF pressure may also theoretically put the repair site at risk as well as other potential complications so any apparent IH needs to be brought to the attention of the treating specialist.
The neural tract is mechanically linked throughout the body. Compromises of neural mobility in body areas remote from the CSF leak site may presumably also impact dural mechanics and function in the spinal and cranial regions as is witnessed in multiple crush syndrome (Butler 2000).
Management of remote mechanical neural restrictions and optimisation of the mechanics of the entire neural tract may therefore improve the longevity of the CSF leak repair and overall recovery of neural function.
Additionally, patients who have undergone patching of a spinal CSF leak have often had substantial volumes of blood or other products introduced into their epidural space.
At least temporarily, this presumably has mechanical impacts on the cord and other surrounding structures, as well as a probable inflammatory response in the dura, potentially generating acute intra-dural and extra-dural pathology at the site of the procedure.
A study of labelled blood injected at lumbar sites for patients in a lateral decubitus position (presumably on a level surface) showed an average spread of 8–10 spinal segments for a patch of 14.8 millilitres with blood spreading more cephalad than caudally and also tracking circumferentially around the epidural space (Szeinfeld et al 1986).
The position in which the patient was placed during and immediately following an EBP procedure will presumably influence the spread of the injected blood and the relationship of the dura and neural tract to both the injected blood and other tissue interfaces, with potential implications for neural mechanics and function in the recovery phase.
Factors believed to substantially increase mechanical load on the dura, and therefore potentially put a repair site at risk, include tension applied axially (such as during spinal flexion or straight leg raise) or transversely, to which the dura is structurally more susceptible than to axial tension (such as during upper limb neurodynamic testing procedures, and spinal lateral flexion) (Breig 1978), and Valsalva-type manoeuvres, which can increase CSF pressure (such as during lifting or straining to empty the bowels) (Prabhakar et al 2007).
These forces will need to be controlled appropriate to the stage of recovery to protect the repair site and facilitate optimal tissue repair.
Body position relative to gravity will impact the position of the cord and dura within the canal, and its relationship to adjacent structures.
For example, in neutral spinal positions the cord will generally move within the canal under the influence of gravity, dropping against the lower side of the canal (eg, the posterior surface when supine, anterior when prone).
Spinal flexion against gravity, however, can add sufficient tension to lift the cord and dura off the lower surface of the canal (Breig 1978).
Body position relative to gravity can therefore presumably impact the mechanics of cord and dura relative to surrounding tissue interfaces during functional and therapeutic movements, and sensible manipulation of this variable may facilitate therapeutic processes (Breig 1978).
Spinal rotation may physically stress the dura, and the presence of impaired segmental control at any level of the spine may also presumably lead to increased mechanical strain on the dura, as persistent rotation of one spinal segment relative to the next has been shown to impart significant stretch to nerve roots (Breig 1978).
An optimal recovery after treatment for a CSF leak will require consideration of the secondary implications of the often-delayed diagnosis and repair. Patients may have experienced significant levels of pain over an extended period and therefore experience central neurological changes related to the ongoing pain input.
In addition, many patients will have experienced repeated recurrence of the leak after an apparently successful patch, making them aware and understandably potentially fearful of the real risk of ‘re-leaking’ with some bodily movements.
Significant deconditioning will also be a reality for many patients who have been severely limited in their ability to function in upright postures for an extended period.
Finally, with much yet to be understood regarding the fluid dynamics of the central nervous system (CNS) and roles of CSF, there may be effects on the CNS function of patients yet to be described as a result of extended periods of reduced CSF volume and pressure, breached dura and the implications of these factors for mechanical protection, immunological and metabolic function of the CNS (Louveau et al 2017).
Future directions
There is much still unknown regarding IH due to spinal CSF leaks, the underlying pathophysiology, effects of treatment procedures, and optimal rehabilitation.
With solid clinical reasoning and the best possible understanding of the factors at play in this condition, physiotherapists can contribute to improving the outcome for these clients through being alert for undiagnosed cases requiring referral, and the provision of sensible, multifaceted management to assist the client‘s optimal recovery.
Further research on multiple fronts should continue to develop our knowledge and clinical practice.
Nicole would like to acknowledge the input of Adjunct Associate Professor David Butler into the research that informs this article. Email inmotion@australian.physio for references. Nicole Frost, APAM, is a musculoskeletal physiotherapist with a keen interest in clinical research. Nicole is the director of Flex-Ability Physio and a past NHMRC grant recipient.
© Copyright 2025 by Australian Physiotherapy Association. All rights reserved.